Creating a cosmetic products could look like making magic : you select some ingredients, you mix them, and you get a wonderful lipstick, a velvety cream or a foamy shower gel.
It’s not so simple, but not so complex neither !
Your first step to uncover the magic, is to meet with 3 ingredients, found in any cosmetic formula : water, oil and surfactant.
Water
You know water, H2O, the first chemical compound you have been in contact with when you are born, and the one you have to drink everyday. Water is everywhere on Earth, and especially in human body.
From a chemical point of view, Water is a polar molecule. As magnets have 2 magnetic poles, water molecules has 2 electric poles (+ & -), due to the specific arrangement of the Hydrogen and Oxygen atoms. Polarity is a key property of water, which explains many of others.
This compound has so many interesting properties in cosmetic formulation :
- Ultra safe : water will not harm your skin
- Good solvent : you can solubilize (i.e. dissolve) plenty of other ingredients in it. These ingredients will be said “hydrophilic” (meaning “loving water”).
- Moisturizing : water by itself brings… moisture to your skin !
- Cheap ! Sorry to say it, but it’s the whole truth : there is not cheaper ingredient than water… and you would be suprised to know the quantity of water in your cosmetic products !
Oil
Rather than “oil”, I should say “oils”. This would include liquid oils (e.g. olive oil), but also “soft” or “solid” oils (e.g. shea butter, paraffin,…etc).
While water is polar, oils are non-polar or far less polar than water. This is due to the structure of oil molecules, classically made of long chains of carbon atoms. This arrangement of atoms distributes electrical charges homogeneously along the oil molecules, avoiding its polarization.
Oils can be identified with 2 caracteristics :
- they leave a greasy / slippery feel on the skin
- they do not mix with water

Oils bring interesting properties to cosmetic formulas :
- emollient : oils make skin soft and supple
- solvent : some interesting ingredients for skin are only soluble in oil, not in water. These ingredients will be said “lipophilic” (meaning “loving oil”) or “hydrophobic” (meaning “hating water”)
- sensory agent : the range of oils, their physical properties (e.g. melting point) is huge. Cosmetic chemist like to combine different oils in their formulas to improve their sensoriality.
Surfactant
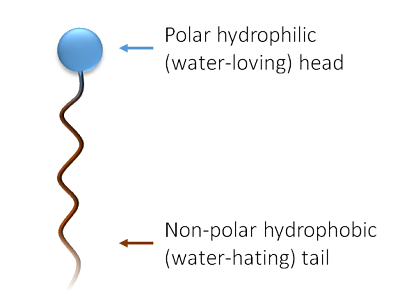
So both water and oil are very interesting for cosmetic formulas, with plenty of benefits for skin and hair. But they do not mix together naturally. That’s where cosmetic chemist use surfactants. Surfactants are molecules that both love water and oil (they are said “amphiphilic”). To achieve this behavior, they have a specific “design” :
When Water, Oils and Surfactants are mixed together, surfactants will go “between” water and oil (one say surfactants will go at the interface between water and oil) :
- the polar hydrophilic part of the surfactant molecule “goes” in water
- the non-polar hydrophobic part of the surfactant molecule “goes” in oils
As science is not so simple, depending on the surfactant used, surfactants will surround either :
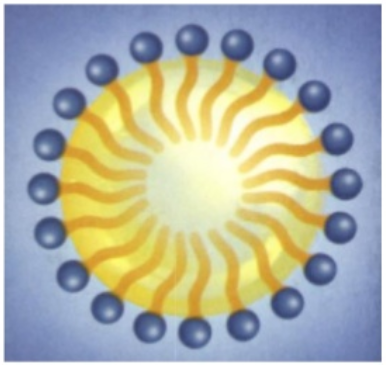
- oil drops within a water environment : we then say we made an “oil-in-water” emulsion :
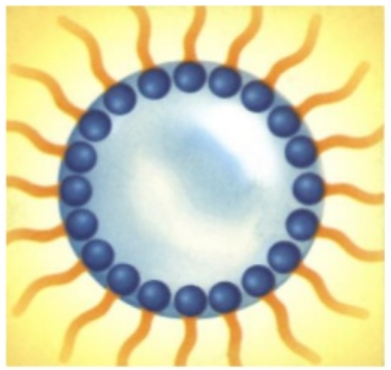
- water drops within an oil environment : we then say we made a “water-in-oil” emulsion :
Emulsion is the classical and essential technology behind any skincare cream and lotion. The combination of water and oils, stabilized with surfactants, brings freshness, moisturization, emolliency, comfort, nutrition to the skin. But cosmetic scientists have much more tricks to make their formulas even more appealing. We will see that very soon !